Every year, companies globally lose up to 2–3 times more money due to non-compliance than they spend on compliance. The impact goes far beyond paying fines. It can slow down production, interrupt supply chains, and damage customer trust built over the years.
For manufacturers, where safety, quality, and consistency are non-negotiable, compliance is more than just following rules. It is about protecting the entire operation. A well-built compliance management system ensures that processes are transparent, minimizes risks, and guarantees that every product leaving the factory meets both regulatory and customer expectations.
Here, you will gain a detailed understanding and practical insights into using a compliance management system to reduce risks and improve productivity.
What is a Compliance Management System in Manufacturing?
A Compliance Management System (CMS) in manufacturing is a structured approach to meeting regulatory, safety, and quality requirements while maintaining accurate and accessible records.
In simple terms, it is the backbone of regulatory adherence in manufacturing. It not only ensures compliance but also creates a culture where following rules becomes a natural part of everyday work.
Unlike generic tools, a manufacturing CMS is specifically designed to meet industry-specific requirements. This includes:
- OSHA safety standards for factory environments
- ANSI and ISO quality management standards
- Material traceability to meet audit requirements
- Regular safety and equipment inspections
Key Benefits of a Manufacturing Compliance Management System
The compliance mistakes can be costly in manufacturing businesses. However, the proper implementation of a compliance management system helps manufacturers to:
- Minimize compliance risks across the plant
- Follow strict rules that protect products, employees, and the environment
- Avoid fines, legal actions, and production stoppages
- Identify potential risks early and prevent them from affecting operations
- Build trust with customers by consistently delivering safe and reliable products
While managing all these factors, a well-managed compliance management system allows manufacturers to focus on growth rather than constantly fixing compliance issues.
Other benefits that come along with the implementation of the right compliance management system include:
Improving Efficiency and Driving Continuous Improvement
A CMS is more than a safety net; it is a tool for smarter, faster operations. It helps you:
- Streamline processes to meet or exceed regulations
- Get a clear view of operations to spot inefficiencies and take corrective actions quickly
- Use continuous monitoring and real-time insights to improve processes regularly
- Increase overall efficiency and productivity across the plant
With the proper CMS, compliance becomes a natural part of operations rather than a separate, time-consuming task.
Managing Data and Staying Audit Ready
Modern compliance management systems rely on digital tools to replace outdated paper-based processes. This brings multiple advantages:
- Centralized Documentation: Keep all records in one secure digital hub for easy access and reduced risk of lost paperwork
- Automated Scheduling: Never miss critical tasks like equipment inspections or safety checks
- Real-Time Tracking: Quickly spot and fix issues before they turn into major problems
- Data-Driven Insights: Analyze compliance data to prevent recurring issues and improve processes
- Audit Readiness: Produce complete and organized records in minutes, saving time and building trust with regulators
A strong CMS transforms complex compliance requirements into simple, actionable workflows. It helps your plant run efficiently, safely, and reliably while giving you peace of mind.
How a Compliance Management System Works for Modern Manufacturing
A compliance management system (CMS) in manufacturing works as the central engine that keeps every process, document, and workflow aligned with industrial regulations and standards.
It connects different operational layers, such as quality, production, safety, and supply chain, to ensure that every activity meets compliance requirements in real time.
Here’s how it works in practice:
1. Centralizing Compliance Data
At the heart of any CMS is centralized documentation. Every record related to compliance—from safety inspections and equipment certifications to material traceability and training records—is stored in one secure digital hub.
This centralized approach ensures:
- Quick access for audits and inspections
- Elimination of missing or misplaced paperwork
- Real-time visibility into compliance status across the plant
By consolidating data, compliance officers can focus on analyzing trends and risks instead of hunting for records.
2. Automating Tasks and Schedules
Manual compliance checks can be prone to errors and time-consuming. A CMS automates critical tasks such as:
- Equipment inspections and calibration schedules
- Safety drills and employee training
- Regulatory reporting deadlines
Automated reminders ensure no task is missed, reducing the risk of non-compliance and freeing staff to focus on higher-value activities.
3. Real-Time Tracking and Monitoring
Modern manufacturing plants are complex, with multiple processes happening simultaneously. A CMS provides real-time tracking of:
- Machine performance and maintenance status
- Safety checks and incident reports
- Supplier compliance and material quality
This continuous monitoring enables managers and compliance officers to identify deviations promptly. For example, if a safety inspection fails on one production line, the system flags it instantly, enabling immediate corrective action.
4. Data-Driven Insights and Analytics
A CMS is not just about collecting data; it is about turning data into actionable insights. Using manufacturing analytics, you can:
- Identify recurring issues and high-risk areas
- Predict potential compliance failures before they happen
- Optimize processes to meet regulatory standards efficiently
- Support strategic decisions for process improvement and resource allocation
These insights enable compliance officers to take a proactive role in managing risk, rather than reacting to problems after they occur.
5. Ensuring Audit Readiness
Audits constitute a significant part of manufacturing compliance, and preparation can be overwhelming without the right system in place. A CMS simplifies this by:
- Organizing all records in one place
- Making them searchable and instantly retrievable
- Generating audit-ready reports within minutes
With a CMS, audit preparation becomes faster and less stressful, allowing teams to demonstrate compliance confidently and maintain trust with regulators and customers.
6. Integrating Across Systems
A modern CMS does not work in isolation. It integrates seamlessly with other systems like:
- ERP systems for production and inventory tracking
- MES (Manufacturing Execution Systems) for process monitoring
- Quality management systems for defect tracking
- Supplier management platforms to ensure upstream compliance
Integration ensures that compliance is part of every business decision, rather than a separate activity.
7. Enabling Continuous Improvement
A CMS supports a culture of continuous improvement by:
- Highlighting inefficiencies or gaps in compliance processes
- Providing feedback loops for corrective actions
- Tracking the effectiveness of implemented improvements
- Helping leadership set measurable compliance goals
Essentially, the system becomes a dynamic framework that evolves with the organization, adapting to new regulations, refining production processes, and mitigating emerging risks.
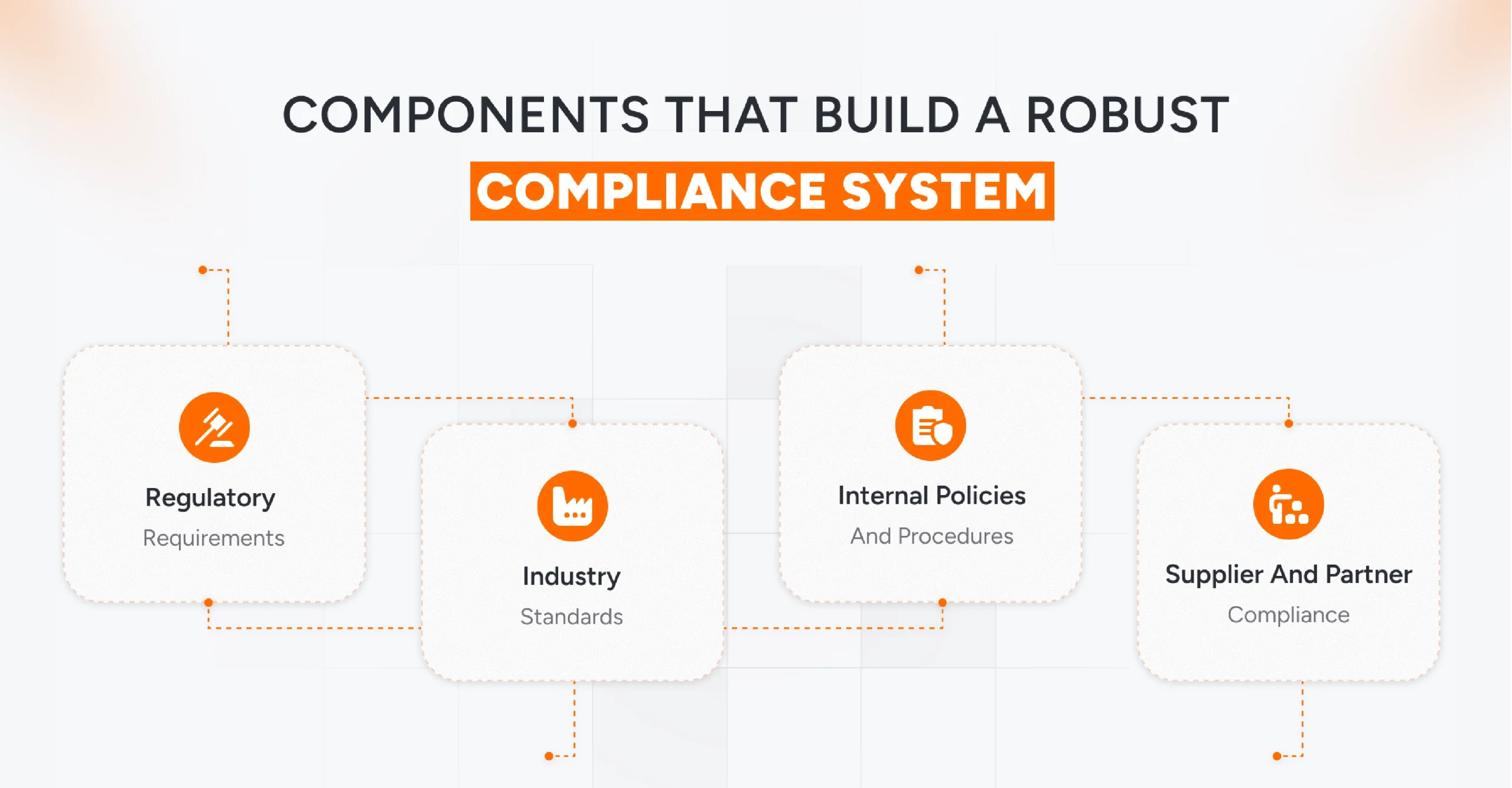
Key Components of a Compliance Management System in Manufacturing
A well-structured Compliance Management System (CMS) in manufacturing integrates multiple layers of governance, operations, and quality control in manufacturing to ensure that every product, process, and partner meets both industry regulations and business standards.
Each component plays a specific role in helping manufacturers maintain consistent, safe, and auditable operations.
1. Regulatory Requirements
Manufacturers operate under strict safety, environmental, and quality regulations, depending on the type of product and region. A CMS helps maintain alignment with these laws by:
- Tracking evolving regulatory standards such as OSHA, EPA, or regional labor and safety norms.
- Ensuring compliance with product-specific mandates, such as or FDA manufacturing requirements.
- Maintaining complete documentation of safety checks, incident reports, and corrective actions.
- Providing automated alerts when any rule, certificate, or document nears expiry or needs renewal.
For manufacturers, this ensures that all activities comply with government and industrial regulations, thereby reducing the risk of costly fines, shutdowns, or safety hazards.
2. Industry Standards
Every manufacturing domain follows recognized frameworks, such as ISO 9001 (Quality Management), ISO 14001 (Environmental Management), ISO 45001 (Occupational Health and Safety), and GMP (Good Manufacturing Practices).
A modern CMS integrates these standards into the workflow to ensure that compliance is not a one-time exercise but a continuous process. It helps manufacturers to:
- Align daily operations with ISO and GMP quality benchmarks.
- Maintain traceability of raw materials and finished products through digital records.
- Conduct regular internal and external audits to measure performance against standards.
- Establish a foundation of a quality-driven culture, where each department contributes to ongoing improvement.
This standardization ensures consistent output, lower rework, and improved customer confidence in manufactured goods.
3. Internal Policies and Procedures
While external regulations set the baseline, internal policies define how a manufacturing company enforces and sustains compliance. Within a CMS, this includes:
- Standard Operating Procedures (SOPs): Digital versions of operational processes to guide workers and ensure uniformity across shifts or locations.
- Employee Training and Certification: Tracking training completion and skill renewals for machine operators, safety staff, and maintenance teams.
- Code of Conduct and Data Protection: Ensuring ethical business behavior and safeguarding sensitive production and employee data.
- Incident and Change Management: Logging and analyzing workplace incidents or process deviations for quick corrective action.
These elements enable to maintain accountability, enhance workforce safety, and foster transparent manufacturing operations management across departments.
4. Supplier and Partner Compliance
Compliance is not limited to internal processes; it extends across the entire supply chain. A CMS ensures that suppliers, vendors, and logistics partners follow the same compliance standards as the manufacturer. It enables:
- Supplier audits and assessments to evaluate safety, environmental, and ethical performance.
- Documentation control for supplier certifications, safety data sheets, and quality records.
- Automated tracking of supplier renewals, product specifications, and contractual obligations.
- Integration with procurement systems for real-time compliance verification before purchase orders are processed.
By maintaining supplier alignment, manufacturers prevent disruptions, maintain quality consistency, and safeguard brand reputation from compliance-related lapses across the supply chain.
Main Features of an Ideal Compliance Management System
A well-built Compliance Management System (CMS) in manufacturing goes beyond checklists. It acts as a digital foundation for maintaining safety, quality, and operational reliability across the plant floor.
The right system seamlessly blends technology with practicality, enabling manufacturers to stay compliant without compromising production. The main features of an ideal compliance management include:
1. Centralized Digital Compliance Hub
A modern CMS brings all compliance data into a single, secure, and accessible space. From material certifications to environmental test results, everything is digitally organized and accessible. This helps manufacturers eliminate scattered spreadsheets and achieve faster traceability during audits or inspections.
2. Dynamic Compliance Scheduling
Instead of relying on manual tracking, automated schedules for equipment calibration, safety inspections, and process reviews keep operations running smoothly. This feature minimizes downtime and ensures no critical activity is ever missed.
3. Smart Policy and SOP Management
The CMS ensures that standard operating procedures (SOPs), work instructions, and safety guidelines are instantly available to the right teams. It supports automatic updates when policies change, helping employees stay up-to-date with the latest regulatory and internal requirements.
4. Intelligent Risk Alerts
Integrated sensors, system triggers, and smart notifications detect early signs of non-compliance. Whether it’s a missed inspection or a temperature variation in a controlled environment, the CMS sends real-time alerts to prevent small issues from becoming major disruptions.
5. Mobile Access and On-Site Tracking
With mobile-ready features, compliance officers and line managers can conduct inspections, record incidents, and upload photos directly from the shop floor. This eliminates paperwork delays and ensures that compliance data is captured accurately in real-time.
6. Digital Sign-Off and Approval Trails
Electronic signatures and automated approval paths make it easy to track who verified what and when. This not only ensures accountability but also makes regulatory reporting smoother and more reliable.
7. Cloud-Based Data Security
A cloud-based security and compliance solution offers encrypted data storage, multi-level authentication, and easy access control. It ensures that sensitive compliance documents are protected while remaining accessible to authorized users from anywhere.
8. Performance Analytics Dashboard
The system’s dashboard converts compliance data into clear insights. Manufacturers can track trends, pinpoint recurring gaps, and measure progress toward compliance goals. This analytical visibility helps leaders make informed decisions and set measurable KPIs.
9. Cross-Department Integration
Seamless integration with ERP, MES, and QMS systems ensures that compliance activities are aligned with production, maintenance, and quality management workflows, ensuring seamless integration. This synchronization eliminates data silos and strengthens overall operational control.
10. Continuous Improvement Engine
An ideal CMS isn’t static. It evolves with every process update, audit report, or new regulation. Continuous feedback loops and AI-driven analytics enable manufacturers to stay aligned with evolving industry standards and regulatory shifts.
The advantages of a well-structured compliance framework often extend beyond regulatory adherence, influencing how teams manage digital content securely and efficiently, as seen in approaches like transforming digital content management with secure SaaS solutions.
Step-by-Step Development and Implementation of a Compliance Management System
Implementing a Compliance Management System (CMS) in manufacturing is not just about meeting regulatory requirements; it’s about building a structured framework that ensures consistency, accountability, and long-term operational excellence. The following steps outline how manufacturers can practically plan, develop, and deploy a CMS that truly adds value.
1. Evaluate the Current Compliance Landscape
Before building or upgrading a CMS, it’s essential to understand your existing compliance environment. This step helps identify where gaps or inefficiencies exist.
Key activities include:
- Conducting a compliance audit to review current safety, environmental, and quality practices.
- Evaluating documentation such as safety inspection logs, supplier certificates, and regulatory submissions.
- Identifying recurring issues like delayed reporting, missed audits, or outdated SOPs.
- Mapping risks that could lead to non-compliance, operational downtime, or product recalls.
This initial assessment provides manufacturers with a clear view of their current strengths and weaknesses, forming the foundation for system design.
2. Define Goals, Scope, and Compliance Objectives
Once the baseline is clear, define what your CMS should achieve. This ensures that the system aligns with both operational and regulatory priorities.
Manufacturers typically:
- Set clear goals such as improving audit readiness, reducing compliance costs, or standardizing practices across plants.
- Define the scope of implementation, including which departments, products, or supplier chains are covered.
- Identify regulatory frameworks relevant to their industry, such as ISO, OSHA, or GMP standards.
- Align CMS objectives with business KPIs, such as reduced downtime, improved quality scores, or faster approval cycles.
3. Design a Scalable and Integrated System Architecture
System design determines how compliance information will flow through the organization. It combines process mapping, role definition, and digital integration.
Manufacturers should:
- Map workflows to define responsibilities and data access levels.
- Integrate the CMS with existing systems such as ERP, MES, and QMS for seamless information exchange.
- Ensure scalability to easily add new production units or product lines.
- Establish centralized dashboards for real-time monitoring and reporting.
This structure ensures that compliance is integrated into daily operations, rather than being treated as an isolated task.
4. Standardize Policies, SOPs, and Internal Controls
Consistency is key to compliance. Every process, from machine maintenance to data recording, should follow standardized policies and procedures.
Manufacturers typically focus on:
- Updating SOPs to match current industry regulations (ISO, GMP, RoHS, REACH, etc.).
- Establishing a code of conduct that promotes ethical and safety-driven operations.
- Implementing uniform training programs across teams and facilities.
- Creating centralized document control to ensure that only the latest, approved versions of procedures are in use.
5. Integrate Digital Tools and Automation
Modern compliance relies heavily on automation to reduce human errors and improve accuracy.
Digital integration may include:
- Automated scheduling for inspections, maintenance, and audits.
- Real-time alerts for license renewals or missed compliance activities.
- Data analytics tools to identify high-risk areas or recurring non-compliance issues.
- Cloud-based document hubs to simplify recordkeeping and access control.
Automation enhances reliability, speeds up responses, and ensures consistent compliance across departments and locations.
6. Train and Empower the Workforce
People are the backbone of compliance success. A well-trained workforce ensures that every procedure is followed with precision.
This stage involves:
- Conducting training sessions on new systems, tools, and reporting procedures.
- Providing role-based access so employees can perform compliance-related tasks effectively.
- Encouraging a compliance-first culture where employees understand the “why” behind every guideline.
- Collecting user feedback to refine system usability and performance.
When employees feel responsible for compliance, adherence becomes a natural and consistent practice.
7. Pilot Testing and Validation
Before the full-scale rollout, manufacturers should test the CMS in a controlled environment to ensure its effectiveness.
Pilot testing helps to:
- Evaluate real-time functionality like data input, alerts, and reporting accuracy.
- Assess system usability for operators, supervisors, and compliance officers.
- Identify potential system bottlenecks and correct them before deploying the organization-wide.
- Validate compliance outputs against existing manual processes to ensure reliability.
A successful pilot ensures that the CMS is both technically sound and practical for day-to-day manufacturing operations.
8. Full-Scale Implementation and Integration
After a successful pilot, the system is rolled out across all departments and facilities.
Implementation steps typically include:
- Phased deployment to minimize disruption to production.
- Integration with supplier and customer portals for end-to-end visibility of compliance.
- Monitoring tools for tracking compliance status in real time.
- Performance dashboards that display metrics such as audit readiness, incident rates, and response time.
This ensures that compliance becomes part of the manufacturing ecosystem, from procurement to final delivery.
9. Continuous Monitoring and Continuous Improvement
Compliance is not a one-time task. Continuous improvement is what makes a CMS truly effective and sustainable.
Key ongoing activities include:
- Regular system audits to ensure accuracy and consistency.
- Periodic updates to match new regulatory changes and global standards.
- Review meetings to analyze performance data and address recurring issues.
- Predictive analytics to forecast potential compliance risks and take preventive action.
This continuous loop ensures that compliance evolves with the business and supports future growth with confidence.
Future Trends in Compliance Management Systems
Manufacturers that stay ahead of emerging trends in compliance can turn regulatory pressure into operational advantage. Below are key developments shaping the evolution of compliance management systems, backed by fresh data, and what they mean in practice.
1. Growing Adoption of AI and Automation
For manufacturers, using AI for risk detection, predictive compliance, and anomaly alerts will become standard. Systems will shift from reactive reporting to proactive monitoring, identifying non-compliance before it impacts safety or product quality.
2. Stronger Demand for Real-Time Monitoring & Visibility
Real-time dashboards, sensors, mobile inspections, and integrated workflows enable issues to be flagged as they occur. It reduces downtime, prevents quality defects, and helps maintain safety continuously rather than waiting for periodic audits.
3. Cloud-Based and Scalable Solutions
Cloud-based CMS offers cheaper deployment, remote access to compliance dashboards, and easier scaling across shifts or multiple sites. It supports collaboration, disaster recovery, and centralized oversight from headquarters to the plant floor.
How AQe Digital Supports Your Compliance Management Journey
AQe Digital helps manufacturers design and implement Compliance Management Systems that simplify operations and meet evolving regulations with precision and ease.
We start by understanding your processes, identifying compliance gaps, and aligning goals with your production environment. Our approach ensures seamless system integration, automation, and long-term sustainability.
Our process includes:
- Assessment & Gap Analysis: Review existing workflows, documentation, and supplier practices.
- Goal Alignment: Define measurable compliance objectives tied to business outcomes.
- System Integration: Connect ERP, MES, and QMS platforms for unified visibility and control.
- Automation & Tracking: Enable alerts, audits, and real-time reporting for seamless operations.
- Team Enablement: Train staff for smooth adoption and consistent usage.
- Continuous Support: Monitor, update, and optimize as standards evolve.
True compliance in manufacturing goes beyond regulations. It defines how responsibly and efficiently an organization operates. A well-defined Compliance Management System brings together people, processes, and technology to create an ecosystem of trust, accountability, and operational excellence.
As manufacturing continues to evolve with digital transformation, the ability to adapt and stay audit-ready becomes a defining edge. Those who integrate intelligent compliance frameworks today are the ones shaping safer, smarter, and more sustainable production for tomorrow.
If your organization is ready to reimagine how compliance drives performance, connect with us to explore a solution designed for long-term growth and resilience.
FAQs
By automating inspections, maintenance schedules, and compliance checks, a CMS prevents equipment failures and process interruptions. Real-time alerts flag potential issues before they halt production, keeping your operations running smoothly.
Yes. Whether it’s ISO, GMP, OSHA, or RoHS, a CMS centralizes all compliance requirements, tracks updates, and ensures your processes meet each standard without duplicating effort or missing deadlines.
The system monitors supplier certifications, audit results, and material traceability to ensure compliance. This ensures incoming materials consistently meet safety and quality standards, reducing rework, rejects, and supply chain delays.
Absolutely. A CMS organizes all records digitally, making it easy to retrieve safety reports, SOPs, and inspection logs instantly. Audits that normally take days can now be completed in hours.
Yes. Modern CMS platforms are scalable. They allow you to onboard new production units, integrate additional equipment, and adjust workflows, ensuring compliance remains seamless as your manufacturing footprint grows.